Our Lab
The Hinton lab work is focused on ATF4 mediated regulation of mitochondrial architecture, lysosomal control, organelle communication, and stress responsive transcriptional networks, thus our research areas are :Signal Transduction and Transcriptional Regulation, Diabetes and Metabolism, and Biophysics and Structural Biology. These three categories most accurately capture the mechanistic, metabolic, and structural dimensions of my research program.
For the updated research description, please use the revised version below:
Dr. Hinton’s research program investigates how ATF4 dependent stress signaling regulates organelle structure, function, and inter organelle communication across physiological and disease states. His laboratory defines how transcriptional control mechanisms coordinate mitochondrial dynamics, cristae remodeling, lysosomal regulation, mitochondrial DNA nucleoid organization, and endoplasmic reticulum mitochondria contact site architecture to control cellular energetics, proteostasis, and metabolic adaptation.
A central emphasis of the lab is understanding how spatial organization and membrane architecture influence mitochondrial signaling capacity. Using advanced three dimensional electron microscopy approaches including SBF SEM, FIB SEM, and CLEM, combined with quantitative morphometric analysis and molecular genetics, the group defines the structural principles governing mitochondrial membrane curvature, cristae density, nucleoid positioning, and organelle distribution within skeletal muscle and other metabolically active tissues.
The laboratory also investigates how ATF4 regulates lysosomal pathways and organelle quality control programs, integrating stress signaling with mitochondrial remodeling and lipid metabolic networks. Through transcriptomic profiling, chromatin occupancy analyses, bioenergetic flux measurements, and computational modeling, the group maps transcriptional programs that couple mitochondrial bioenergetics, redox balance, and adaptive stress responses.
In parallel, the team develops next generation electron microscopy workflows and artificial intelligence driven computational pipelines to classify mitochondrial ultrastructure, membrane topology, and organelle interactions across aging, metabolic disease, and stress conditions. This integrated framework positions ATF4 as a central regulator of organelle plasticity linking signal transduction, metabolism, and structural biology in cardiometabolic, skeletal muscle, and systemic stress related diseases.
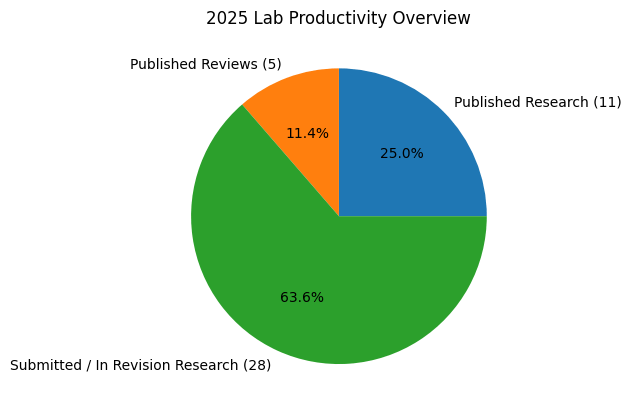
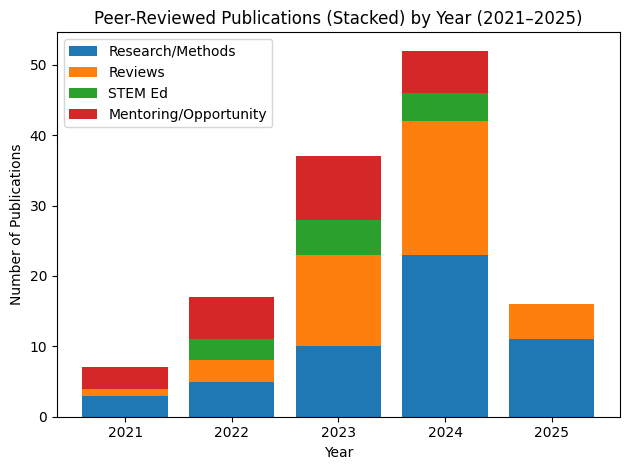
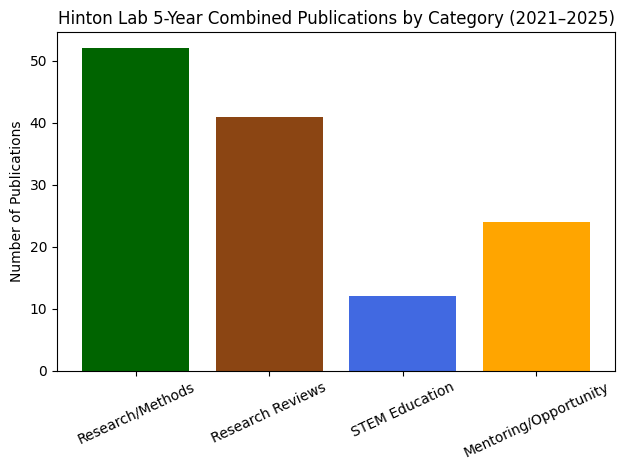
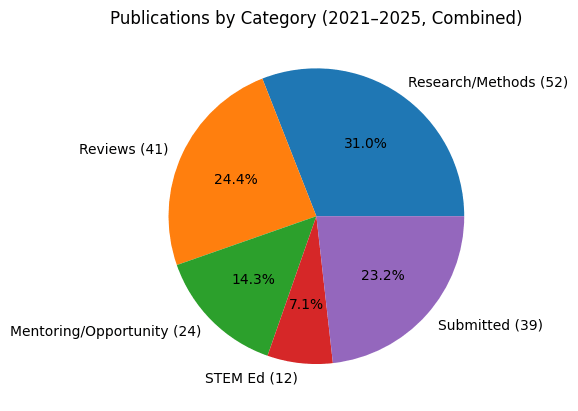
Lab Stats